Lucid & OLCC Compliance
Lucid Design has 5+ years of experience in creating and submitting OLCC/OHA compliant packaging for our cannabis clients. On August 15th, 2018, new OLCC rules will become effective, requiring many changes to the current packaging and labeling currently approved and sitting on shelves. We invite you to take a condensed look at the required changes listed here and contact us if you would like help navigating the compliance for your package or label. Feel free to get in touch with us at any point to look over your packaging and help make adjustments as needed.
What’s Changed
There are several changes and updates to the OLCC’s definitions of current products, changes in labeling and packaging requirements based on cannabis categories, and new rules and corresponding universal symbol for industrial hemp products. Some changes are minor; others are not. You can find the full administrative order HERE. You can find the most updated version of the OLCC Packaging and Labeling Checklist, updated 07/10/18, HERE. To save you time, we’ve condensed and highlighted some of the most important changes.
Updates to Labeling Definitions
The OLCC will now list cannabinoid tinctures and capsules as separate from cannabinoid edibles and this change must be reflected on packaging and labeling.
UPDATES TO TINCTURE & CAPSULE DEFINITIONS
A cannabinoid tincture will now be listed as a liquid cannabinoid product packaged in a container of 4 fluid ounces or less that consists of either:
(a) A non-potable solution consisting of at least 25% non-denatured alcohol, in addition to cannabinoid concentrate, extract or usable marijuana, and perhaps other ingredients intended for human consumption or ingestion
(b) A non-potable solution comprised of glycerin, plant-based oil, or concentrated syrup; cannabinoid concentrate, extract or usable marijuana; and perhaps other ingredients that does not contain any added sweeteners and is intended for human consumption or ingestion
Capsule and Tincture Labeling Requirements
Tincture and Capsule Labels must include the amount, in milligrams or THC and CBD in each serving and in the container.
Other Cannabinoid Products Definition
This is defined as a cannabinoid product that contains two or more ingredients and is not intended for human consumption, including but not limited to products that combine usable marijuana and concentrates or extracts; or usable marijuana, concentrates or extracts that contain added substances.
Hemp Symbol Now Required
The OLCC will soon make a new Hemp symbol available to licensees that will be required for display on all labeling and packaging of industrial hemp commodities or products. An industrial hemp commodity or product is defined as: an item processed by a handler or processor containing any industrial hemp or containing any chemical compounds derived from industrial hemp, including CBD derived from industrial hemp. This definition does not include industrial hemp that has been minimally processed or has not been processed in any form.
Pre-Roll Net Weight Changes
The net weight, when dealing with pre-rolled cannabis, must now include the dried leaves and flowers, the rolling paper, and the filter or tip.
Continually Re-Sealable Packaging Requirement - Child Resistance
If the container holding the marijuana item or industrial hemp commodity or product does not meet the child-resistant standards, the outermost label must contain the following statement: “This package is not child resistant.”
There are new updates to the packaging requirements depending on cannabis categories. For cannabinoid products, concentrates and extracts: All containers and packaging must be packaged in a container that is resealable and continuously child-resistant. For usable marijuana: All usable marijuana must be child resistant for a least a single use.
Cannabinoid Labeling Updates – New Requirements
A label must now contain all required information in any typed, legible font that is easy to read and contrasts sufficiently with the background. All fonts must be at least 1/16th of an inch in height based on the the uppercase K. A good example of this size would be the letter ‘K’ in Arial at 6.25 font.
Label Identification
Once a label is approved by the Commission, the label identification number provided must be prominently displayed on the label of the outermost container.
Product Identity
Product identity (aka, edible, extract, tincture) must be in bold type, in a size reasonably related to the most prominent printed matter on the principal display panel. The identity must be parallel to the base on which the package rests as it is designed and displayed. The product identity for extracts and concentrates must correctly identify whether the product is one or the other labeled in accordance to type.
Net Quantities
The net quantity must be a distinct item separated from other printed label information on all sides by at least a space equal to the height of the typeface used in the net quantity. Net quantity should be displayed in bold type in the bottom 30 percent of the principal display panel and in lines generally parallel with the base of the container.
Lab Names
The testing information on cannabinoid products must include the name of the lab that performed any test—Lab Identification numbers are no longer required. For medical registrants registered with the Oregon Health Authority, the label must contain the name of the lab that performed any test, any associated test batch number and any test analysis date for the final product.
Motor Vehicle Warning Update
Required warning now updated as ‘Do not drive a motor vehicle while under the influence of marijuana.
Added Substances
If a cannabis extract or concentrate contains any added substances, the item shall be considered a cannabinoid product and labeled as such.
THC & CBD Percentage Fluctuations
THC and CBD amounts vary based on the value calculated at the laboratory. These amounts are allowed to vary plus or minus up to 10% percent of the specified recreational limitations.
Small & Tiny Container Label Requirements
Labels which, due to size, does not have sufficient space for a label that contains all the information required must adhere to these rules:
(A) A principal display panel containing the net weight or volume, product identity, and universal symbol
(B) Licensee business or trade name and license number or registrant business or trade name and registrant number
(C) UID number
(D) Concentration or amount of THC and CBD in the container; and
(E) Required warnings.
(i) For a retail marijuana item or industrial hemp commodity or product, the following warning is required on the label: “For use only by adults 21 and older. Keep out of reach of children.”
Tiny container Labels are defined as a container that has a complete surface area available for applying a label that is less than 2 inches squared. Tiny container labels must include:
(A) A principal display panel with the universal symbol and product identity;
(B) UID number;
(C) Concentration or amount of THC and CBD in the container;
(D) Licensee or registrant business or trade name and license or registrant number; and
(E) A warning that reads: “Keep out of reach of children.”
Ingredients List Updates
The list of ingredients must correctly identify the type of marijuana item or industrial hemp ingredient used to make the product.
New Nutritional Labeling Formats
Edible packaging must use one of the nutrition information formats provided by the OLCC to display required information. Edible labeling must include the amount of calories, sodium, protein, added sugars, cholesterol, total carbohydrates, and total fat per serving, in grams or milligrams as appropriate.
Industrial Hemp Product Labeling
All principal display panels must contain the new hemp symbol. Labels must contain the following warnings: ‘This product is derived from hemp and could contain THC. Keep out of reach of children.’
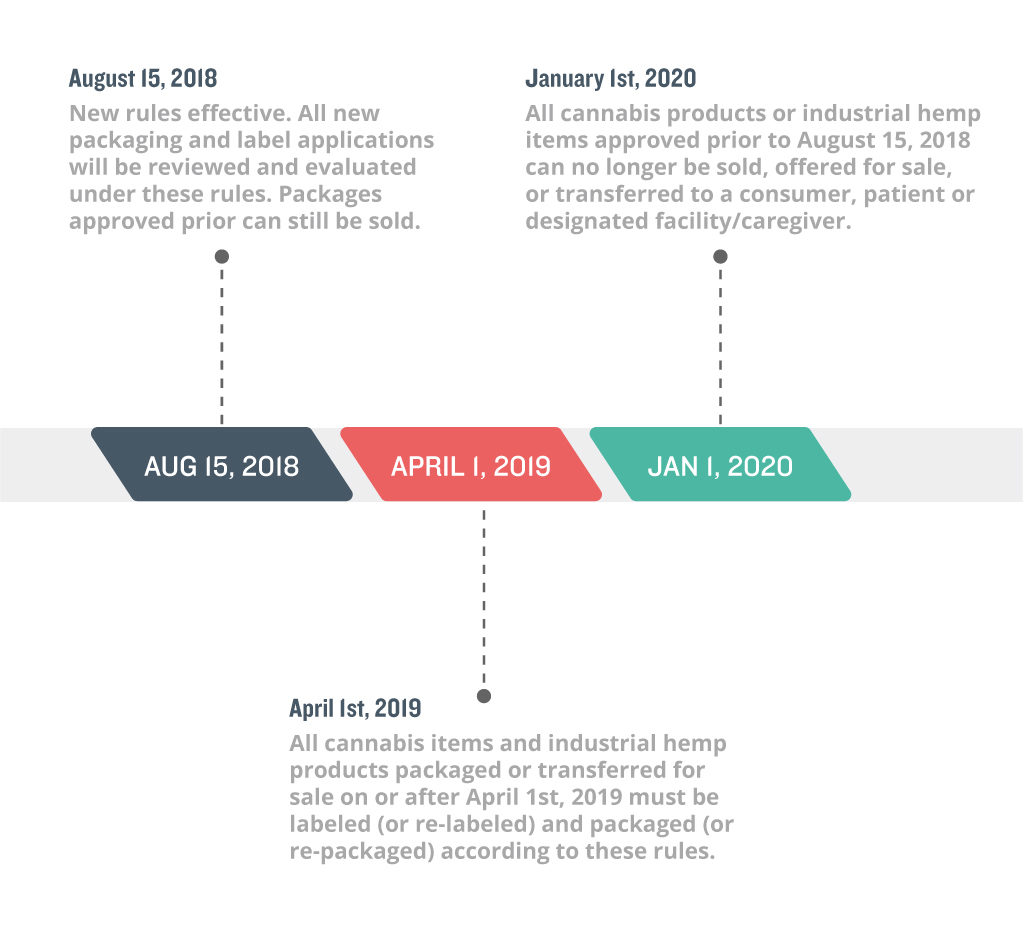
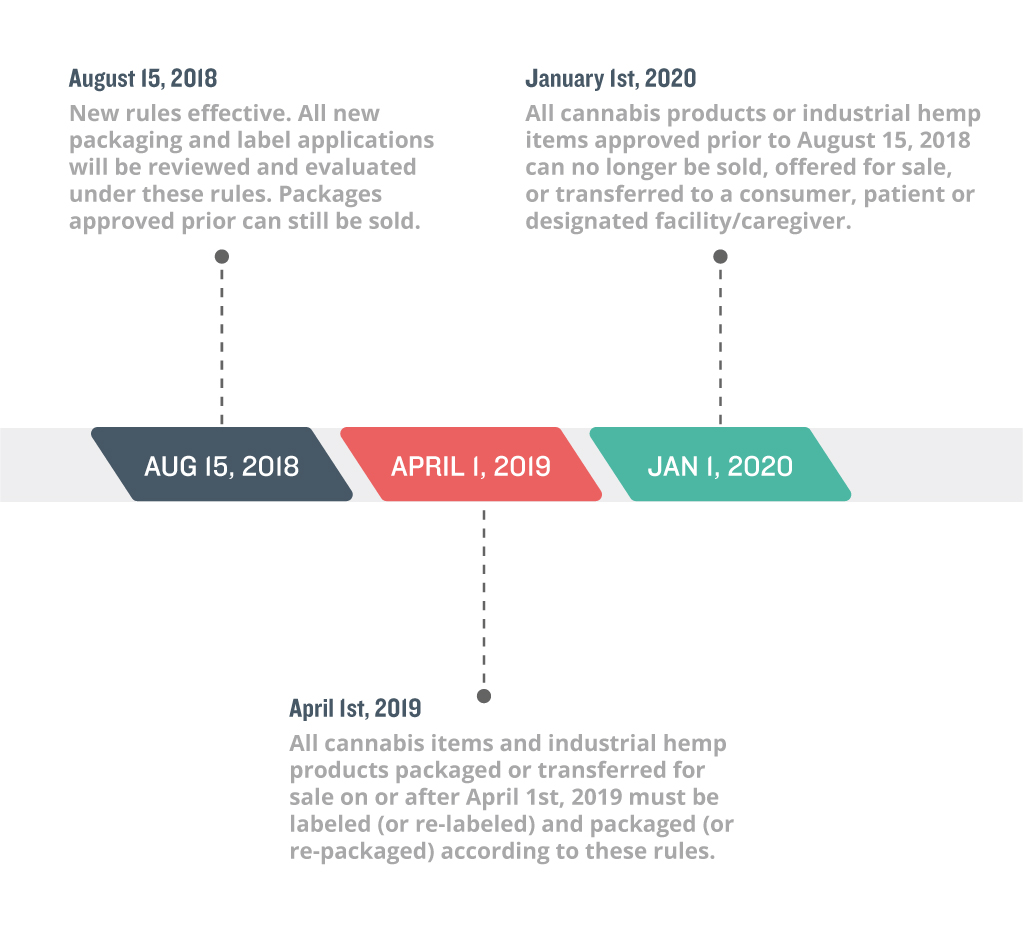
Compliance Timelines
These rules become effective on August 15, 2018. All package applications submitted past August 15th, 2018 will be reviewed and evaluated under the new changes. For registrants with packaging and labeling already approved, all products currently in the METRC system may continue being sold ‘as-is’, as long as they have been transferred for sale to a registered dispensary by March 31st, 2019. All products and packaging created, submitted or OLCC approved on or after April 1st, 2019 must adhere to the new regulations. By January 1st, 2020, all packaging and labels created, submitted or approved prior to August 15, 2018 can no longer be sold by unless they are repackaged relabeled in adherence to the new rules.
Please contact us here or email info@lucid-design.com with any packaging or compliance questions. You may also call our office, 503-241-0539.
